Release time:Oct 25, 2023
ESMO Congress 2023 was held in Madrid, Spain from October 20-24, the preliminary results of the Phase I/II study of 9MW2821 (Nectin-4 targeting ADC) in patients with advanced solid tumors were reported by Dr. Jian Zhang of Fudan University Shanghai Cancer Center, on behalf of the research team. The results of the Phase III study of 8MW0511 were presented as a poster.

9MW2821: Oral Presentation
Background
Nectin-4 is an adhesion molecule that highly expressed in variety of solid tumors including urothelial cancer and could be a potent therapeutic target. 9MW2821 is a monoclonal antibody-drug conjugate (ADC) that delivers monomethyl auristatin E to cells expressing Nectin-4. The study assessed its safety, tolerability and preliminary efficacy in patients with solid tumors.
Methods
9MW2821 was administered by intravenous infusion on days 1, 8 and 15 of each 28-day cycle. The study included dose escalation, dose expansion and cohort expansion period which included urothelial cancer (UC) and other Nectin-4 positive solid tumors. Primary objectives were assessment of safety and preliminary efficacy.
Results
As of April 27, 2023, 97 patients (including 39 UC patients and 29 cervical cancer patients) were enrolled with doses ranging from 0.33 to 1.5mg/kg. Median age was 57 years (range, 32-78). All patients have been treated with platinum-based chemotherapy and immune checkpoint inhibitors before enrollment.
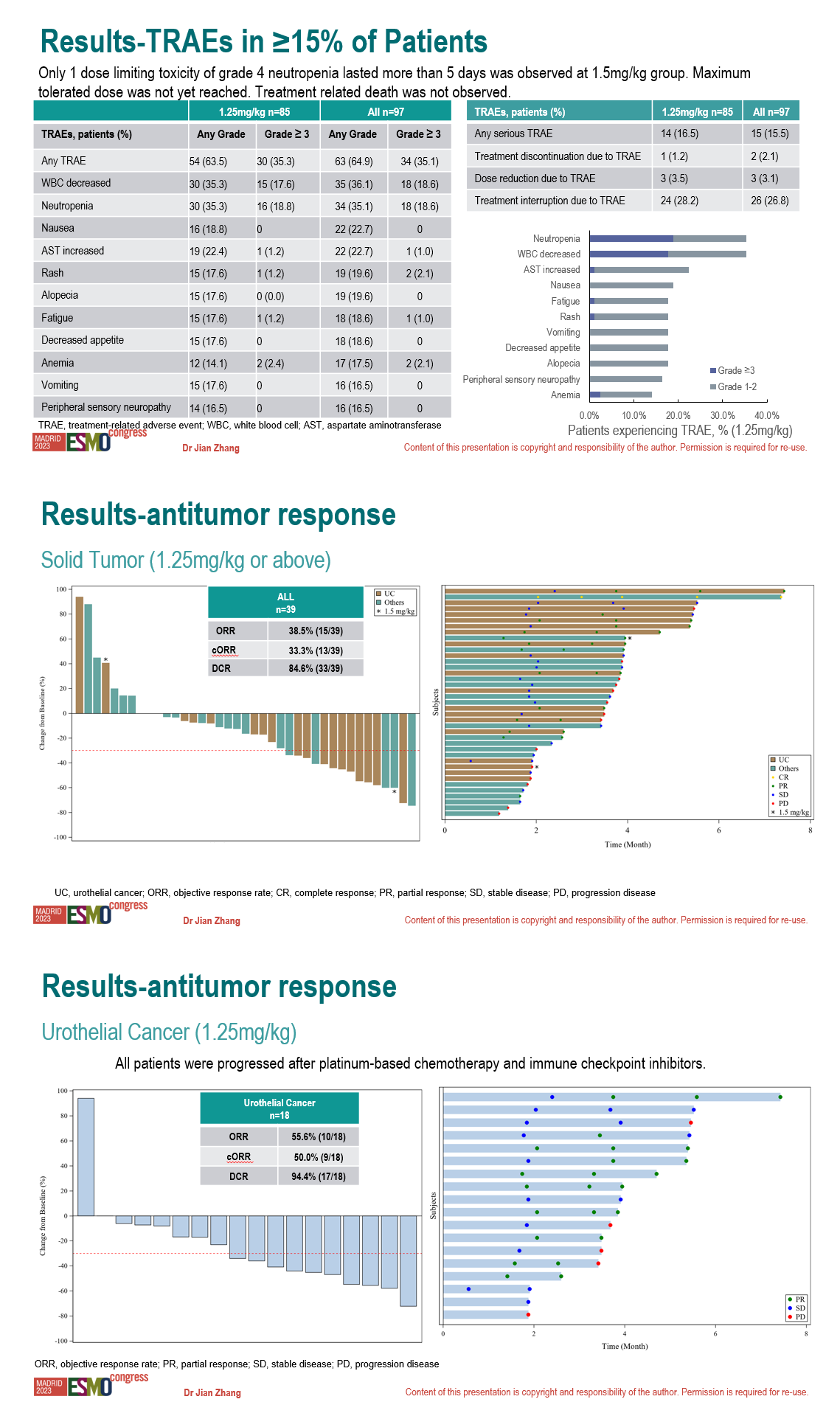
Treatment related death was not observed. Only 1 dose limiting toxicity of grade 4 neutropenia lasted more than 5 days was observed at 1.5mg/kg group. Maximum tolerated dose was not yet reached.
Treatment related adverse events (TRAEs) of any grade occurred in 64.9% pts. The most common TRAEs were white blood cell (WBC) count decreased (36.1%), neutropenia (35.1%), nausea (22.7%), aspartate aminotransferase increased (22.7%), rash (19.6%), alopecia (19.6%), fatigue (18.6%), decreased appetite (18.6%), anemia (17.5%), vomiting (16.5%), peripheral sensory neuropathy (16.5%). Grade 3/4 TRAEs occurred in 35.1% pts. The most common grade 3/4 TRAEs were WBC count decreased (18.6%) and neutropenia (18.6%).
Among 39 subjects with solid tumor who treated with 9MW2821 at 1.25mg/kg or above and evaluable for tumor assessment, ORR and DCR was 38.5% and 84.6%, respectively. In 18 patients with UC who dosed at 1.25mg/kg and evaluable for tumor assessment, ORR and DCR was 55.6% and 94.4%, respectively. Objective responses were also observed in patients with breast cancer and cervical cancer.
Conclusion
9MW2821 showed manageable safety profile. Hematological toxicity, the most common adverse events associated with 9MW2821, were deemed to be manageable, tolerable and reversible.
In addition to urothelial cancer, 9MW2821 showed promising antitumor activity in multiple tumor types.
Enrollment continues to determine efficacy of 9MW2821 in certain solid tumors.
8MW0511: Poster
Phase III study of recombinant (yeast-secreted) human granulocyte-colony stimulation factor fusion protein 8MW0511 for injection reported at ESMO showed that 8MW0511 was clinically effective, non-inferior to the positive control. It is able to improve the incidence and duration of grade 4 neutropenia, with a significantly lower incidence and duration of grade 4 neutropenia observed at cycle 2-3 than in the positive control group. The overall safety profile is similar to that of the positive control group, which indicates manageable safety profile and good tolerance in humans.