Release time:Feb 19, 2024
Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, recently published the phase III study results of denosumab biosimilar (MW032) online in the international top journal of JAMA Oncology. This is the first recorded trial to systematically compare the efficacy, population pharmacokinetics, and safety profile of MW032 and reference denosumab in patients with solid tumors with bone metastasis through a 53-week, multicenter, randomized, double-blind, phase 3 equivalence trial.

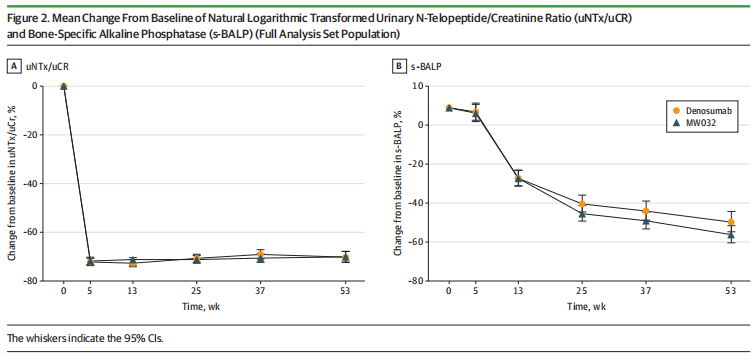
708 eligible patients were enrolled and randomly assigned (1:1) to receive MW032 or reference denosumab subcutaneously every 4 weeks until week 49. The primary end point was percentage change from baseline to week 13 of natural logarithmic transformed urinary N-telopeptide/creatinine ratio (uNTx/uCr). Secondary end points were percentage change in uNTx/uCr and bone-specific alkaline phosphatase (s-BALP) from baseline to weeks 5, 13, 25, 37, and 53, and the incidence of SREs.
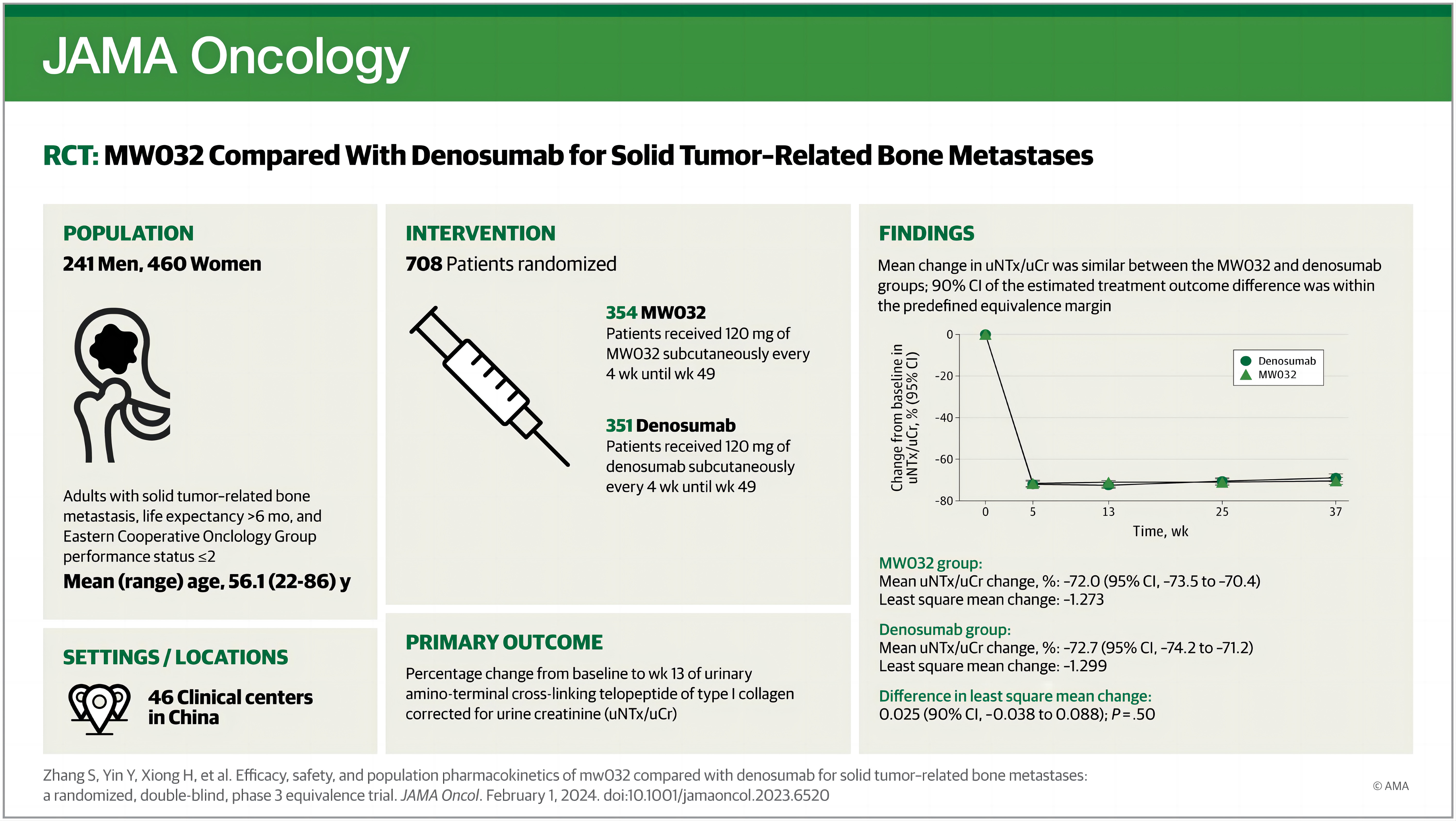
The results showed that the mean change of uNTx/uCr from baseline to week 13 was ?72.0% in the MW032 group and ?72.7% in the control group. After adjusting for stratification factors, the LSM (least squares mean) difference of these logarithmic transformed percent changes was 0.02. The 90% CI for the difference (?0.04 to 0.09) was within the equivalence margin. Sensitivity analyses of the primary end point, subgroup analysis, and secondary end points analysis demonstrated similarity in efficacy between the two treatment groups.
705 patients receiving at least 1 dose of investigational therapy were included in the safety analyses. The incidence of adverse events (AE), treatment related AEs, and AEs leading to discontinuation were similar in the MW032 and control groups. Treatment related AEs with the highest incidence were hypocalcemia, hypophosphatemia, and hyperuricemia, which occurred at similar rates in both treatment groups. Similarity study of immunogenicity and population pharmacokinetics have been conducted in the study.
The study systematically demonstrated that MW032 and denosumab were biosimilar in efficacy, safety profile and population pharmacokinetics. Launch of MW032 will broaden patient access to denosumab, reduce financial burden on patients, and finally benefit more patients with advanced tumors.
Find paper at:https://jamanetwork.com/journals/jamaoncology/fullarticle/2814859